Are you looking for Questions with answers for Quality control job interview preparation? I’m going to give you some tips from this post to help you rock your interview. It makes no difference if you are being interviewed for a position as a quality control officer, executive, or manager. you can obtain the list of Interview Questions for Quality Control, I advise you to study In order to assist you to pass your QC interview, I will then provide you with some excellent examples of interview replies. Additionally, I will outline the qualities you must exhibit throughout your Quality Control interview. Prior to answering the Quality Control interview questions, I always do this by providing you with excellent, original responses that you won’t find anywhere. Let’s get started with some recommendations for quality control interviews, questions, and answers.
Quality control in pharmaceuticals involves the testing of all new or existing products. It becomes important to know the questions related to quality control to get a dream job in the quality section. If you are a graduate in pharmacy or a master in science, then before the interview you can read all the Interview Questions for the Quality Control department so that you get confident about How to answer Quality control interview Questions.

Important Interview Questions for Quality Control Analyst
Question: What exactly is Quality control?
Ans: The word quality control refers to the collection of all techniques used to assure the identification and purity of a certain pharmaceutical product. It includes analyzing a pharmaceutical product’s chemical, physical, and sometimes microbiological properties.
Pharmaceutical products are tested for quality control against specifications.
Other tasks of Quality Control include raw and packaging material sampling, testing of raw and packing material, in-process, finished product, and stability batches. Water sampling and testing, instrument calibration, preparation of specifications for raw, packing, in-process, and finished products, preparation of standard test procedures for raw, packing, in-process, and finished products, and reporting of results after analysis and preparation of COA are all part of the task.
Question: Some important Full Forms used in QC:
ISPE: International Society of Pharmaceutical Engineering
ASTME: American standards of testing and material
ICH: international council on harmonization
EMA: European medicinal agency
HSA: Health Science Authority
SAP: System Application And Product in Data Processing
SCM:: System Chain Management
RMG : Rapid Mixer Granulator
SMG: Saizoner mixer granulator
RLAF: Reverse Laminar Air Flow
LAF: Laminar Air Flow
VTS: Vacuum transfer system
HVAC: Heating, Ventilation, and Air Conditioning
AHU: Air Handling Unit
HEPA: High-Efficiency Particulate Air filter.
ULPA: Ultra-Low Penetration Air
VMP: Validation Master Plan
LOAEL: Lowest Observed Adverse Effect Level
NOEL: No Observable Effect Level
PDE: Permitted Daily Exposure
ADI: Acceptable Daily Intake
MAR: Maximum allowable residue
MACO: Maximum Allowable Carryover
PIC/S: Pharmaceutical Inspection Co-Operation Scheme
CTD: Common Technical Document
MSDS: Material Safety Data Sheet
BOPP: Biaxial Oriented Polypropylene
MPD: Master packing documents
CPV: Continues Process Validation
CPP: Critical Process Parameters
CQA: Critical Quality Attribute.
SUPAC: Scale –Up and Post approval changes
SUPAC IR: Immediate release
SUPAC MR: Modified release
Certification & Standards of ISO:-
ISO 9001:2015 – Quality Management System
ISO 14001: 2015 – Environment Management System:
ISO 45001:2018 – Occupational Health & Safety Management System
Common Quality Control Interview Questions:
If you are looking for QC interview questions for freshers as well as for experience, Then below are the Questions that are always used by interviewers during interviews in Pharmaceuticals.
Question: Tell me About the Disintegration Test:
Ans: It is the system that involves the Tablet / Capsule breaking into particles; the disintegration test is a measurement of the time required for a group of tablets/capsules to disintegrate into particles under a certain set of conditions (Temperature).
The maximum cycle rate of the shaft holding the tube basket is 29-32 cycles per minute, the distance covered by the shaft basket is 50-60 mm, and the beaker temperature is 35 to 39 °C.
Disintegration is to be performed to determine if tablets or capsules dissolve in the specified period when immersed in a liquid medium under the test conditions.
Question: Define pH. What is the pH of the blood?
Ans: pH is the negative logarithm of H+ Concentration. The pH of the blood ranges from 7.35 to 7.45.
Question: Explain the terms Aliquot and Diluent.
Ans: Aliquot: Aliquot is a measured sub-volume of the original sample.
Diluent: the component used to dilute the sample.
Question: Explain what is Titration.
Ans: Titration is also called volumetric analysis. It is a quantitative chemical analysis used to determine the concentration of an analyte that has been identified. The titrator is a reagent that is prepared as a standard solution with a known concentration and volume. The titrant reacts with the analyte solution to determine the concentration of the analyte. The titration volume is the amount of titrant that reacts with the analyte.
Question: Types of Titration?
Ans: There are basically four types of titration, acid-base titration, complexometric titration, precipitation titration, and redox titration.
Question: Explain the four types of titration
Ans: Acid-base titration: this acidic or basic titrant reacts with an analyte that is a base or an acid.
Complexometric titrations: involving metal-ligand complexation reactions
Precipitation titrations: When the analyte and titrant react, a precipitate is formed.
Redox titrations: Where the titrant is oxidizing agents or reducing agents.
Question: What is Karl Fischer’s Titration?
Ans: Karl Fischer titration is a classic titration method in chemical analysis that employs coulometric or volumetric titration to identify trace quantities of water in a sample. Karl Fischer, a German scientist, created it in 1935.
Question: What is meant by the solution?
Ans: A solution is a mixture of liquids, gases, and solids, the solution consists of many different types of solutes like salts, oxygen, and organic molecules.
Question: Describe the Saturated and Unsaturated solutions
Ans: A saturated solution is defined as a solution in which a solvent is not capable of dissolving any more solute at a given temperature.
At a given temperature, an unsaturated solution is one in which the solvent is capable of dissolving any extra solute.
Question: What is the difference between qualitative and Quantitative analysis?
Ans: Qualitative analysis involves the identification of the compound or chemical based on its chemical ( absorption, emission) or physical properties, eg. melting point and boiling point.
Quantitative analysis: this involves the estimation or determination of the concentration or amount of the chemical compounds or components.
Read Also: Top Pharma Interview Questions for Freshers
Interview Questions for Quality Control For Different Spectroscopy Techniques:
Question: Explain the principle of Ultraviolet Spectroscopy.
Ans: Ultraviolet spectroscopy uses light in the UV part of the electromagnetic spectrum. UV absorption spectra form when the outer electrons of a molecule or an atom absorb energy and move from a lower to a higher energy level. The wavelength absorbance of each molecule is unique.
Question: What is The HPLC Principle?
Ans: It’s a technology used for separating the mixture of compounds into individual components based on absorption, partition, ion exchange, and size exclusion principles. The stationary phase and mobile phase are used in it. HPLC is used for the identification, quantification, and purification of components from a mixture.
Question: What is thin-layer chromatography?
Ans: Thin layer chromatography (TLC) is a chromatographic technique used to separate and analyze compounds in a mixture. It involves the use of a thin, flat, and stationary phase (such as a glass plate coated with a thin layer of an adsorbent material) and a mobile phase (liquid solvent). As the mobile phase moves over the stationary phase, the components of the mixture separate based on their affinity for the stationary phase. TLC is used for both qualitative and quantitative analysis of samples.
Question: What is Infrared spectroscopy?
Ans: Infrared spectroscopy is a technique used to study and identify chemical substances by measuring their interaction with infrared radiation. This interaction can occur through absorption, emission, or reflection of infrared light. Infrared spectroscopy is valuable for analyzing the functional groups and chemical bonds present in solid, liquid, or gaseous samples.
Question: What is the range of infrared spectroscopy?
Ans: Infrared spectroscopy covers a range of wavelengths from 700 to 1000 nanometers (nm) in terms of wavelength or 14,286 to 12,800 per centimeter (cm-1) in terms of wavenumber. Ultraviolet radiation has wavenumbers above these values, typically ranging from 25,000 to 50,000 cm-1 or 100 to 400 nm in wavelength.
Question: What is the Ultraviolet (UV) and visible spectroscopy range?
Ans: The range of UV Spectroscopy is 200-400 nm, and visible spectroscopy ranges from 400- 800 nm.
Question: What is the use of UV Spectroscopy?
Ans: Spectroscopy can be used to detect functional groups, and impurities, and perform qualitative and quantitative analyses.?
Quality Control Technician interview questions:
Question: Define Molarity.
Ans: A number of moles of solute per liter solution. molarity is denoted with a capital “M”.
Question: Define molality.
Ans: The number of moles of solute per kilogram solvent. it is denoted with a small “m”.
Question: Define normality.
Ans: The number of moles equivalent per liter solution.
Question: What are buffer solutions?
Ans: A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. When a minimal amount of strong acid or base is introduced to it, the pH changes very little.
Question: What is valency?
Ans: Valency is simply the combining power of elements. the valency determines the chemical formula of a compound. When compounds react to form new compound (s) they tend to change their valences.
Question: What is aqua regia?
Ans: A mixture of concentrated nitric acid and hydrochloric acids. (1:3) It’s a highly corrosive liquid that can harm gold and other hardened materials.
Question: What is the bleaching powder’s chemical name?
Ans: Calcium hypochlorite, also known as CaOCl2, is an inorganic chemical compound having the formula CaOCl2. Calcium oxychloride is another name for them.
Question: What is polarity?
Ans: Polarity is the electronegativity difference between the atom or molecule or the ability of an atom to attract shared electrons in a covalent bond. Water is a good example of a polar molecule due to the difference in electronegativity between the oxygen atom and the hydrogen. Oxygen is a highly electronegative atom compared to hydrogen. Because fats, petrol, and gasoline do not dissolve in water, they are classified as non-polar molecules. Nonpolar means “insoluble in water.”
Question: Explain the Beer Lamberts Law.
Ans: It states that the intensity of nonchromatic light absorbed by a substance dissolved in a fully transmitting solvent is directly proportional to the substance concentration and the path length of the light through the solution.
Question: What is an indicator in chemistry?
Ans: Indicators are substances that show a change in color when brought in contact with acid or base. The most commonly used indicators are litmus, methyl orange, and phenolphthalein which change color as follows.
| Indicator | Acid Solution | Basic Solution | Neutral Solution |
| Blue litmus solution | Red | No Change in Color | No Change in Color |
| Red litmus solution | No Change in Color | Blue | No Change in Color |
| Methyl Orange | Red | Yellow | Orange |
| Phenolphthalein | Colorless | Red | Colorless |
Top Interview Questions for Quality Control in Manufacturing
Question: Explain the Infrared Spectroscopy Principle.
Ans: When a molecule absorbs infrared radiation, it vibrates and gives rise to a packed infrared absorption spectrum. This IR spectrum is specific for every different molecule absorbing the IR radiation, useful for identification.
Question: What is Gas Chromatography?
Question: What is the common alum?
Ans: Potassium alum, potash alum, or potassium aluminum sulfate is a chemical compound Chemical formula of common alum is KAI(SO4)2.12H2O. Use water purification.
Question: What is a covalent bond?
Ans: A covalent bond also called a Molecular bond is a chemical bond that involves the sharing of electron pairs between atoms.
Question: Mention the formula to calculate the pH of a solution.
Ans: Formula to calculate pH = -log [H+] or pH = -log [H3O+]
Question: What is PPM?
Ans: PPM is parts per million (such as % means parts per 100)
Que
Question: Explain what is dextro-rotatory and levorotatory.
Ans: Levorotation and Dextrorotation are referred to as the properties of plane-polarized light when light rotates clockwise when it approaches the observer then known as dextro-rotatory, and when light rotates anticlockwise then it’s referred to as levorotatory.
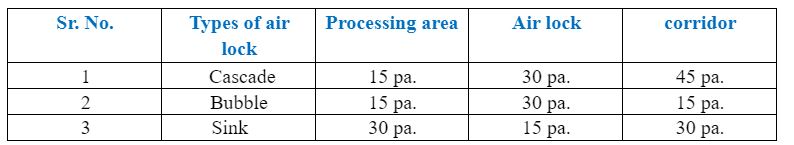
Question: Tell me different types of Air lock systems (DP)
Ans:
Question: What is OOS?
Ans: Out of Specification (OOS) results are those results, generated during testing that do not comply with the relevant specification or standards or with the defined acceptance criteria.
Question: What is OOT and define it?
Ans: A. “OOT” stands for Out Of Trend. It means any test results obtained for a particular batch that is markedly different from the results of the batches in a series obtained using the same validated method.
Question: What is 5-point pH calibration?
Ans: A 5-point pH calibration is a process where a pH measuring instrument is calibrated using standard buffer solutions at five different pH values. In this case, the calibration is performed using buffers with pH values of 1.68, 5.01/5.00, 7.00/7.01, 10.00/10.01, and 12.45. This calibration ensures the accuracy and reliability of the pH measurements taken by the instrument across a wide pH range.
Question: What is pharmacopoeia?
Ans: A pharmacopoeia is an official and legally binding collection of standards and quality specifications for medicines used within a specific country or region. It provides guidelines for the quality, purity, strength, and labeling of pharmaceutical substances and dosage forms. Pharmacopoeias are crucial for ensuring the safety and efficacy of medicines.
Question: What are the types of pharmacopeias?
Ans: There are several types of pharmacopeias, including:
- United States Pharmacopeia (USP)
- British Pharmacopoeia (BP)
- European Pharmacopoeia (Ph. Eur.)
- International Pharmacopoeia (Ph. Int.)
- Japanese Pharmacopoeia (JP)
- Indian Pharmacopoeia (IP)
- Chinese Pharmacopoeia (ChP)
Question: What is Solubility?
Ans: Solubility refers to the maximum concentration at which a substance can dissolve in a given solvent at a specific temperature and pressure. It is an essential property in various fields, especially in drug discovery and development, as the solubility of a compound can significantly impact its pharmacological properties and bioavailability.
Question: What is Dissolution?
Ans: Dissolution refers to the process of a solid substance, typically a pharmaceutical tablet or capsule, dissolving in a liquid (usually a solvent) under controlled conditions. It measures the rate at which a drug is released from its dosage form and becomes available for absorption in the body. Dissolution testing is essential for assessing the drug’s bioavailability and ensuring its effectiveness.
Question: What Is Analytical Method Validation?
Ans: Analytical Method Validation is the process of providing documented evidence that an analytical method is suitable for its intended purpose. It ensures that the method is reliable, accurate, and consistent in generating results that meet specific quality standards.
Question: What are the Parameters of Method Validation?
Ans: Parameters involved in method validation include:
- Selectivity and specificity
- Linearity
- Range
- Accuracy
- Precision
- Limit of quantification
- Ruggedness
- Robustness
Question: Quality Risk Management Methodology
Ans: Basic risk management facilitation methods (flow charts, check sheets, etc.)
Failure Mode Effects Analysis (FMEA)
Failure Mode, Effects, and Criticality Analysis (FMECA)
Fault Tree Analysis (FTA)
Hazard Analysis and Critical Control Points (HACCP)
Hazard Operability Analysis (HAZOP)
Preliminary Hazard Analysis (PHA)
Risk ranking and filtering
Supporting statistical tool
Question: What is the formula for calculating the number of air changes in an area?
Ans: The number of air changes/hour in an area is = Total Room Air-flow In CFM x 60
Total Volume of room in cubic feet.
For calculating Total Room Airflow in CFM, first, calculate the air flow of individual filters.
The formula is given below.
Airflow (in CFM) = Avg. velocity of air (in feet / Minute) x Effective area of
the filter. Then find the Total airflow.
The formula is total air flow = Sum of air flow of the individual filter.
Airflow Velocity can be measured with the help of an Anemometer.
Note: More Questions based on “Quality control manager interview tips” will be updated soon. So keep visiting to read and explore more.
Conclusion:
These Questions are usually very important for usually, those who are preparing for interviews in the quality control (QC) section in pharmaceuticals. Hope these Interview Questions for Quality Control with answers will be helpful. if any updates or doubts please reply below.
This is a typical quality control interview question, and it is typical because conflict can occasionally arise in quality control because some individuals see the quality control process as a barrier. And as a result, you must be able to settle disputes quickly and decisively. Here is my recommendation: “I would approach disputes head-on and work to find a fast solution. Conflict of any kind can be harmful to an employee and the company. In order to resolve the problem amicably, it is crucial to identify its underlying causes. In order to attempt to determine what the issue was, I would chat privately with the party with whom the conflict was occurring.
In order to determine whether the behavior was causing the disagreement and If the source of the problem was a disagreement with our organization’s quality control procedures, I would start by educating the other party on why QC was so essential. I would give concrete instances of how quality control can improve a company’s success as well as how it can give workers and staff a stronger sense of job satisfaction knowing that the high-quality work they were producing benefited the team and the larger company as a whole. So that shows that you’re not afraid to deal with conflict, but you will deal with it professionally and amicably and also with a view to educating the person in respect of how quality control can have a positive impact within an organization.
Note: All above Answers to Interview Questions for Quality control will be updated from time to time whenever changes happen in methods or formulas.

Plse upload more questions that asked in interview.
Sure, Thank you!