Standard operating Procedure for Issuance, Use, and Retrieval of Punches and Dies and it is most important to maintain their inventory in pharmaceuticals.

1.0 Objective: To establish a procedure for the issuance, use, and retrieval of punches and dies.
2.0 Scope: This procedure covers the issuance, use, and retrieval of punches and dies used in the production department.
3.0 Responsibility: Officers and Executives in the Production Department
Managers in the Production Department
4.0 Procedure:
4.1 Issuance of Dies and Punches:
4.1.1 Issue the required number of dies and punches for a specific product according to the respective Batch Manufacturing Record (BMR).
4.1.2 Prior to assembly, inspect all tooling for damage. Report any deviations to the department head and QA.
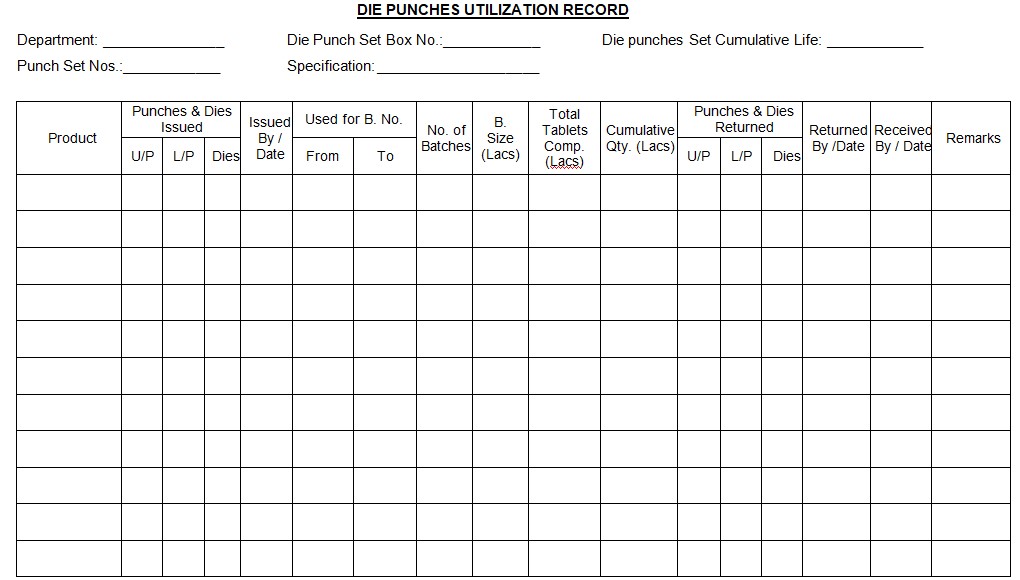
4.1.3 Record the details in the Punches and Dies Utilization Record (refer to Annexure-I).
4.2 Use of Dies and Punches:
4.2.1 Utilize punches and dies on a rotation basis.
4.3 For 37-station compression machines:
4.3.1 First issue: Use subsets 1 to 37.
4.3.2 Subsequent issues: Rotate subsets to ensure uniform utilization of all spare subsets.
4.4 For 35-station compression machines:
4.4.1 First issue: Use subsets 1 to 35.
4.4.2 Subsequent issues: Rotate sub-sets for uniform utilization.
4.4.3 Mark ‘✔’ for issued subsets and ‘—’ for non-issued subsets in Annexure-II.
4.4.4 Follow the correct tooling instructions from the BMR.
Related SOP: SOP on Operating Procedure for 35-Station Double Rotary Compression Machine
4.5 Retrieval of Dies and Punches:
4.5.1 After batch completion, remove dies and punches from the compression machine.
4.5.2 Clean punches and dies according to SOP PRD/041.
4.5.3 Transfer cleaned punch sets to the tool room and inspect them for defects per “Punch and die inspection” SOP.
4.5.4 Record entries in Annexure-I for retrieved good dies and punches.
4.5.5 If abnormalities are found, isolate the product and punch set, placing it on hold.
4.5.6 Inform the Production and QA Incharge.
4.5.7 Investigate the incident cause and prepare an action plan for further processing.
4.5.8 Reject the punch set after compressing five million tablets per subset.
5.0 Abbreviations:
BMR: Batch Manufacturing Record
QA: Quality Assurance
6.0 Annexures:
ANNEXURE – I: Punches and Dies Utilization Record
Related SOP: SOP for Polishing of Punches and Dies