Standard Procedure for Transferring Finished Products (After Product Packing Completion) to Finished Goods Stores via Proper Procedure.

1.0 Objective: This procedure aims to establish a clear process for the transfer of finished products to the finished goods stores.
2.0 Scope: This procedure is applicable to the transfer of finished products to the finished goods stores within the production department.
3.0 Responsibility:
Officer, Executive – Production Department
Manager – Production Department
In charge – Stores
4.0 Definitions:
Not Applicable (NA)
5.0 Procedure:
5.1 Upon completing the packaging, reconcile the packed quantity with the quantity received from manufacturing and stores.
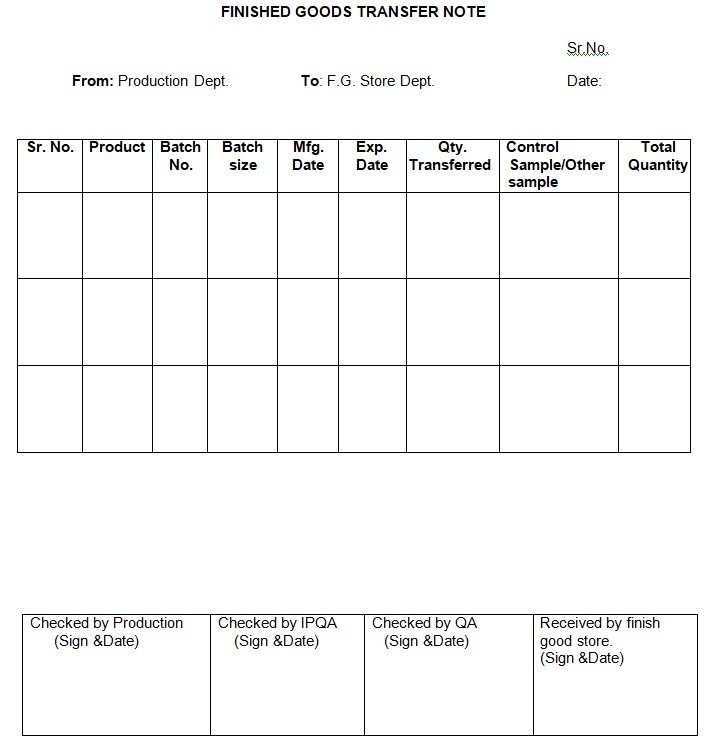
5.2 Generate a finished goods transfer note in triplicate, following the format in Annexure-II.
5.3 If the quantity of packs is lower than indicated on the shipper label, label the last shipper of the batch as a ‘LOOSE BOX.’ Cross out the preprinted quantity on the label, replace it with the actual quantity, and have the production officer and QA officer sign the label.
5.4 Notify the Quality Assurance (QA) department to inspect the packed goods before transferring them to the finished goods stores.
5.5 The QA Officer is responsible for verifying the quantity and shipper labeling of the finished drug product.
5.6 Once QA verification is complete, transfer the finished drug product to the finished goods stores.
5.7 The store officer / in charge must verify the quantity stated on the transfer slip and then proceed with the transfer to finished goods stores.
5.8 The store officer / in charge should endorse the transfer slip and return the book to the packing department.
5.9 Record the transfer slip number in the respective Batch Packing Record (BPR).
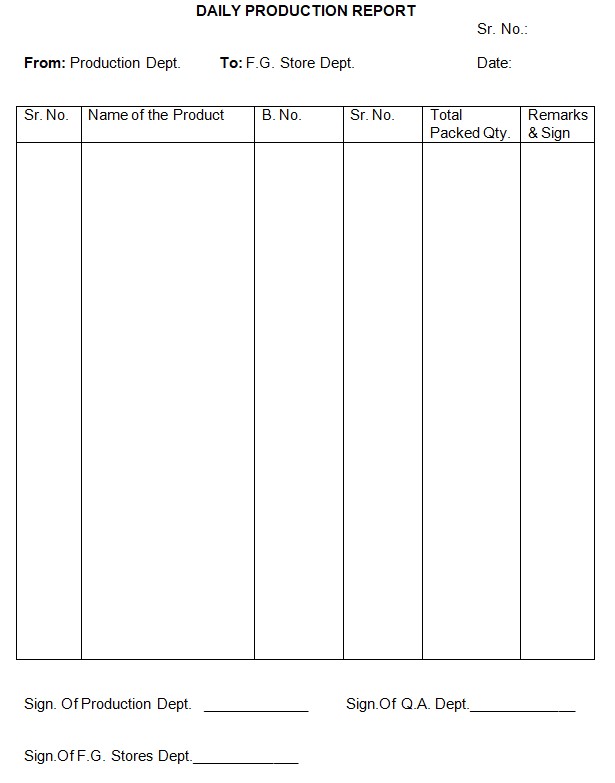
Note: This procedure is also applicable as an end-of-day activity, as outlined in Annexure-I.
Related SOP: SOP on Making entries in equipment usage and cleaning log sheet
6.0 Abbreviations:
BPR: Batch Packing Record
QA: Quality Assurance
7.0 References:
Not Applicable (NA)
8.0 Annexures:
Annexure-I: Daily Production Report
Annexure-II: Finished Goods Transfer Note
Related SOP: SOP on Movement of Materials from one stage to another