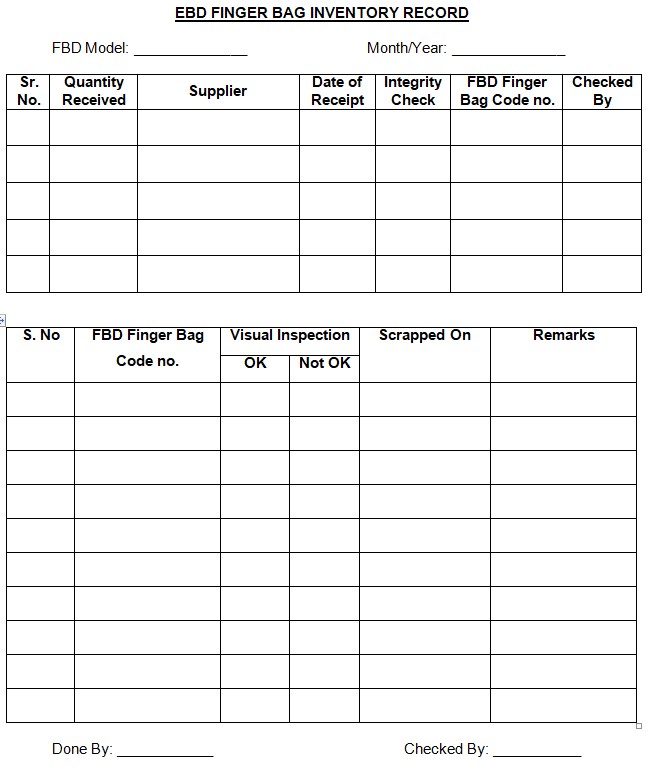
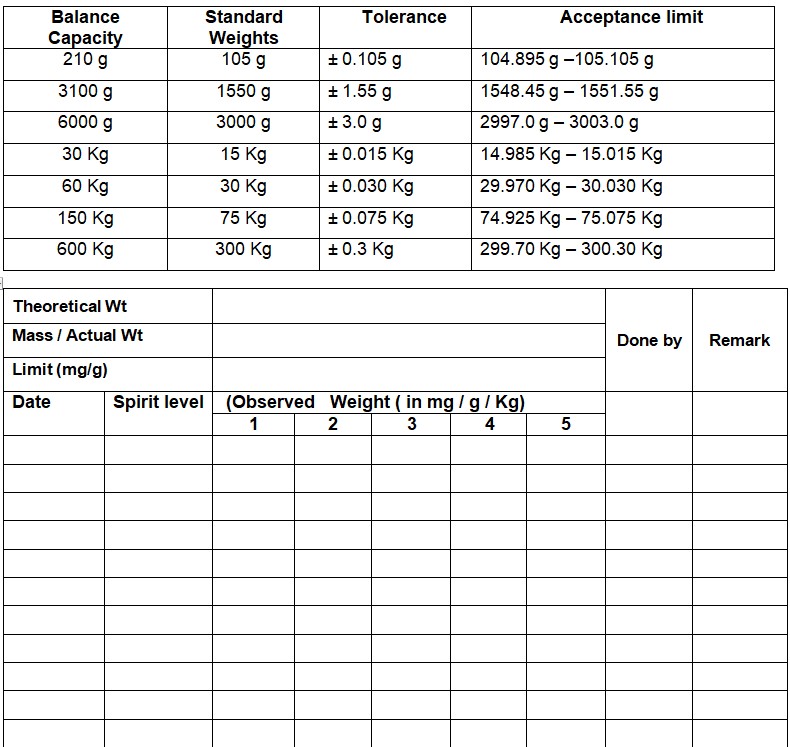
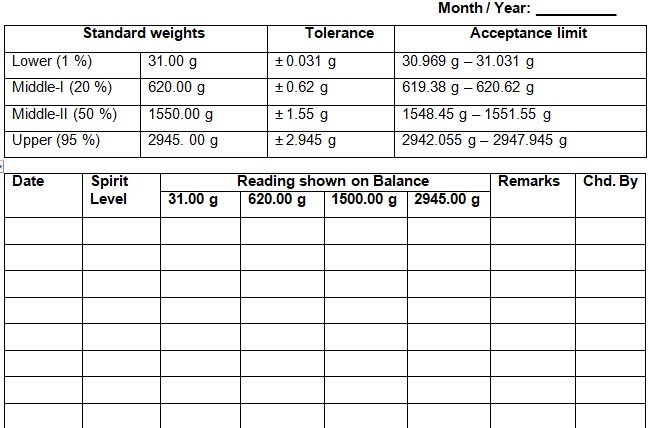
SOP on Cleaning of SS / HDPE containers, Inprocess containers (IPCs), Blender Bins, pallets, new sieve/screen and accessories
1.0 OBJECTIVE: The objective of this procedure is to establish guidelines for effectively cleaning SS/HDPE containers, in-process containers (IPCs), blender bins, pallets, new sieves/screens, and accessories. 2.0 SCOPE: This procedure applies to the cleaning process of SS/HDPE containers, in-process containers (IPCs), blender bins, pallets, SS items, new sieves/screens, and accessories within the production department. 3.0 … Read more